Introduction
Currently, there is no cure for Alzheimer’s disease, a progressive neurodegenerative condition that affects millions of people worldwide. However, treatments are available to help manage its symptoms, such as memory loss, confusion, and difficulties with thinking and reasoning. While these therapies do not stop the disease, some have shown promise in slowing its progression, offering hope for improving the quality of life for those affected.
Alzheimer’s is characterized by the abnormal accumulation of proteins in the brain, such as beta-amyloid plaques and tau tangles, which interfere with the transmission of nerve impulses and disrupt normal brain function. These proteins build ups damage and ultimately kill brain cells, leading to the cognitive and functional decline associated with the disease.
In a groundbreaking study, researchers tested a synthetic peptide on mice genetically engineered to develop Alzheimer’s-like symptoms. The findings were promising, showing that the treatment significantly reduced the buildup of these harmful proteins in the brain. Even more compelling, the peptide therapy appeared to restore key cognitive functions, such as memory and learning, which are typically impaired in Alzheimer’s patients.
This study written about below highlights the potential of innovative therapeutic approaches to target the root causes of Alzheimer’s, rather than just addressing its symptoms. While further research, including human trials, is necessary to validate these findings, this development represents a significant step forward in the quest for more effective treatments and, ultimately, a cure for Alzheimer’s disease.
The problem
As global life expectancy continues to rise, dementia has become an increasingly pressing public health challenge. The prevalence of dementia is projected to grow significantly, with studies estimating that over 150 million people worldwide could be affected by 2050. This staggering statistic underscores the urgent need for effective prevention and treatment strategies.
Alzheimer’s disease, the most common form of dementia, is characterized by a variety of debilitating symptoms, including memory loss, cognitive decline, and personality changes. These symptoms are widely believed to result from the accumulation of two key proteins in the brain: beta-amyloid (Aβ) and tau. Beta-amyloid forms sticky plaques between neurons, while tau creates tangles within neurons, disrupting their structure and function. Together, these protein abnormalities lead to widespread neuronal damage, brain shrinkage, and the hallmark symptoms of Alzheimer’s.
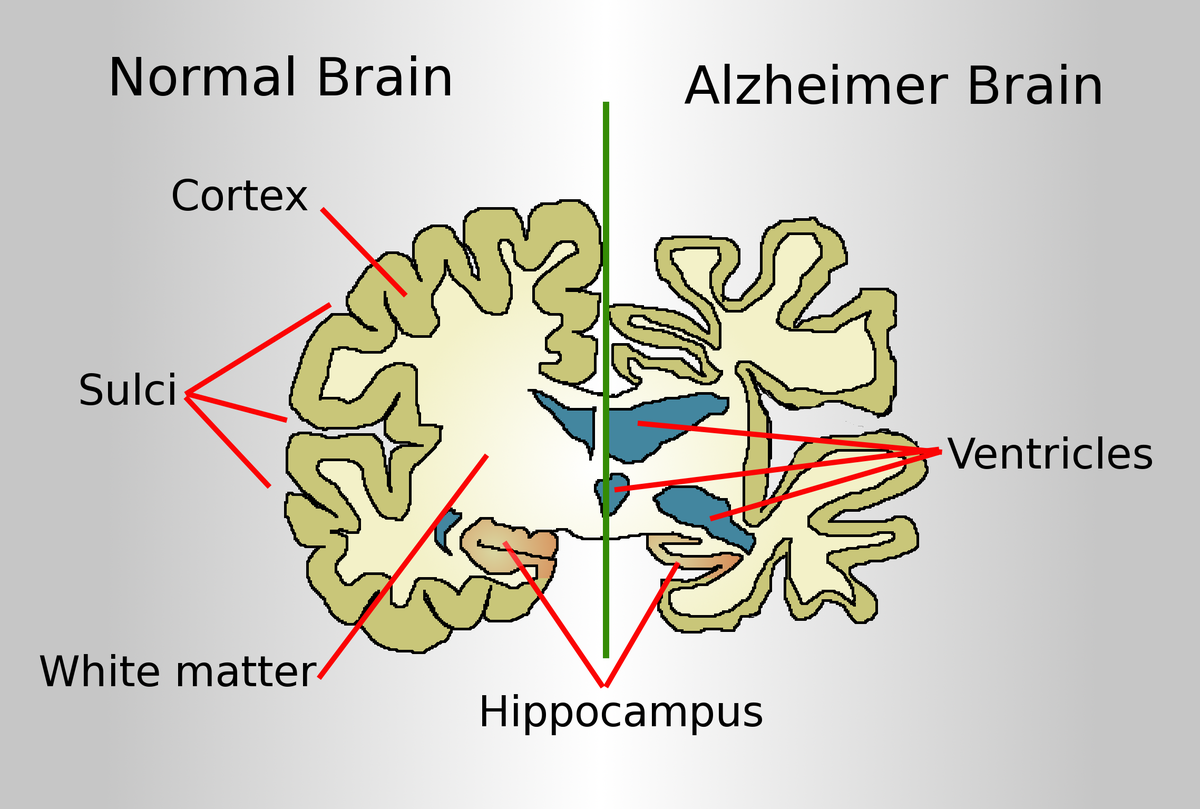
Current treatments for Alzheimer’s primarily focus on alleviating symptoms, such as cognitive deficits and behavioral changes, rather than addressing the underlying causes of the disease. However, recent advancements have introduced disease-modifying therapies that aim to directly target these pathological proteins. For example, aducanumab and lecanemab, two monoclonal antibody treatments, have shown promise in reducing Aβ plaques in the brain. These therapies mark a significant step forward in targeting the disease at its root.
Despite these advancements, the use of monoclonal antibody treatments has sparked debate within the medical community. While they offer the potential to slow disease progression, their benefits are tempered by notable risks. Common side effects include brain swelling and microhemorrhages, raising concerns about the overall safety and efficacy of these treatments. Some experts question whether the modest clinical improvements observed in patients justify the associated risks, particularly considering the complex, multifactorial nature of Alzheimer’s disease.
As the global burden of dementia continues to rise, ongoing research is essential to refine these treatments, explore alternative therapeutic targets, and better understand the mechanisms underlying Alzheimer’s. The goal is to develop safer, more effective interventions that can not only slow disease progression but also prevent its onset, offering hope to millions of individuals and families affected by this devastating condition.
The possible solution
A recent study has introduced a promising new approach to treating Alzheimer’s disease by targeting tau protein accumulation. Tau proteins, when abnormally modified, form neurofibrillary tangles that disrupt the passage of nerve impulses across synapses, the critical junctions where nerve cells communicate. These tangles are a hallmark of Alzheimer’s and contribute significantly to the cognitive and memory impairments associated with the disease.
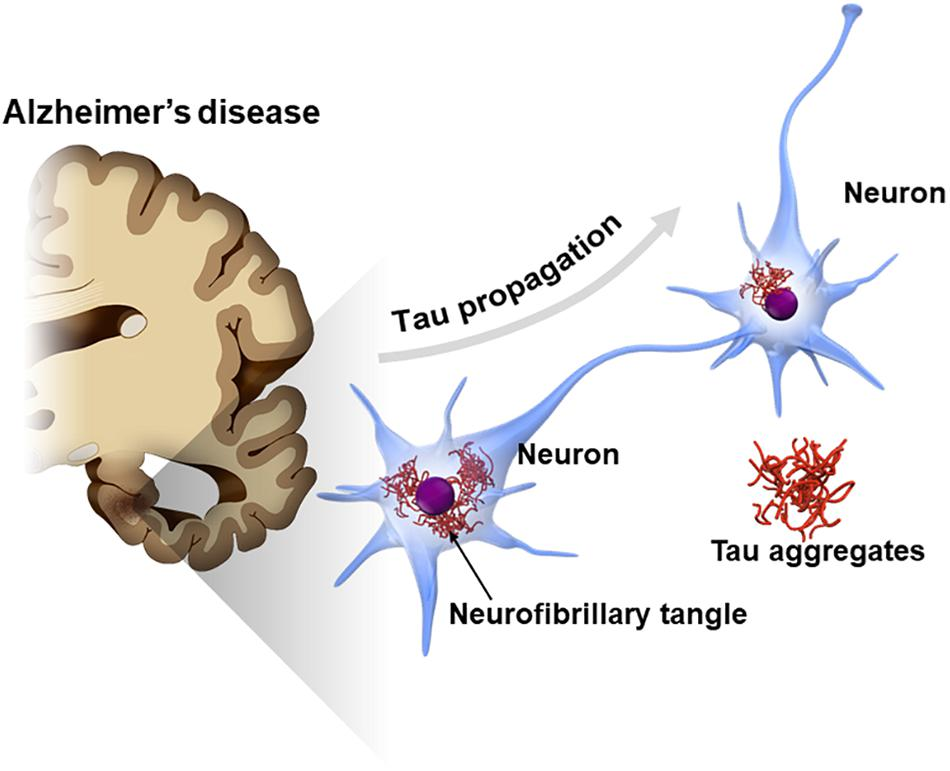
In this research, scientists tested the effects of a synthetic peptide, PHDP5, on transgenic mice engineered to develop Alzheimer’s-like symptoms. The peptide was found to inhibit a specific biochemical pathway responsible for tau buildup, effectively preventing the formation of these neurofibrillary tangles. Remarkably, the treatment not only reduced tau accumulation but also reversed deficits in memory and learning in the mice, suggesting its potential to restore cognitive functions impaired by Alzheimer’s.
The findings, published in the journal Brain Research, represent a significant step forward in understanding and addressing the complex mechanisms underlying Alzheimer’s disease. Unlike existing treatments that primarily target beta-amyloid plaques, this study highlights the importance of tau as a therapeutic target. By focusing on tau, the researchers offer a complementary approach that could work alongside beta-amyloid-targeting therapies to provide a more comprehensive strategy for tackling the disease.
Dr. Stefania Forner, the Alzheimer’s Association director of medical and scientific relations, who was not involved in the study, emphasized the significance of the findings. “Using a mouse model of Alzheimer’s disease, this study sheds some light on a novel potential treatment pathway,” she stated, underscoring the innovative nature of this research.
The use of PHDP5 as a therapeutic agent offers several advantages over traditional approaches. Targeting tau tangles directly could address a broader range of Alzheimer’s symptoms, particularly in patients where beta-amyloid-targeting therapies have shown limited efficacy. Additionally, the peptide’s ability to reverse memory and learning deficits provides hope for interventions that not only halt disease progression but also restore lost cognitive abilities.
Peptide reduces tau buildup in brain
The transmission of nerve impulses across synapses—the communication hubs between nerve cells—is a highly coordinated process that depends on two key components: dynamin and microtubules. These structures work in tandem to facilitate the recycling of vesicles, which are tiny sacs that store and release neurotransmitters. Neurotransmitters are essential chemicals that carry the nerve impulse from one neuron to another, ensuring seamless communication in the nervous system.
In healthy individuals, tau proteins play a critical role in stabilizing microtubules, which serve as the structural framework of nerve cells. This stability ensures that dynamin remains available to support vesicle recycling and maintain effective synaptic communication. However, in Alzheimer’s disease, tau proteins become dysfunctional. They detach from the microtubules, leading to their destabilization. This process also results in the removal of dynamin from nerve cells, disrupting the recycling of vesicles. Over time, the detached tau aggregates into tangles, a hallmark of Alzheimer’s disease, further impairing the transmission of nerve impulses and contributing to cognitive decline.
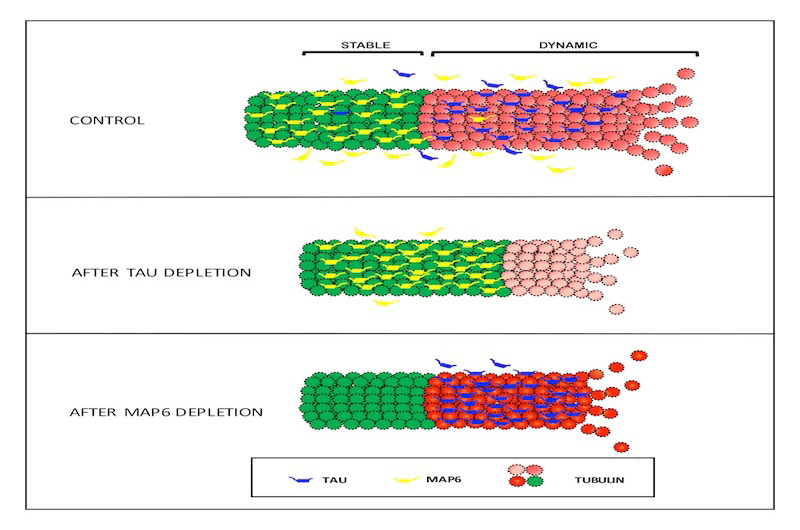
In earlier in vitro (laboratory-based) research, scientists explored the potential of a synthetic peptide, PHPD5, to counteract these damaging effects. The peptide was shown to release dynamin, making it available to support the recycling of vesicles and restore communication between synapses. By targeting this critical pathway, PHPD5 effectively bypassed the disruption caused by tau dysfunction, offering a novel mechanism to preserve synaptic health.
Building on these promising in vitro findings, recent studies have expanded to animal models, where PHPD5 demonstrated the ability to reverse memory and learning deficits by addressing tau-related disruptions. By stabilizing microtubules and freeing dynamin for vesicle recycling, the peptide restores a fundamental aspect of neuronal function that is severely compromised in Alzheimer’s disease.
This research examines the complexity of Alzheimer’s pathology and highlights the need for multifaceted therapeutic approaches. While much of the focus in Alzheimer’s treatment has traditionally been on targeting beta-amyloid plaques, these findings reinforce the critical role of tau in disease progression. By addressing tau-induced disruptions in synaptic communication, PHPD5 offers a complementary strategy that could work synergistically with beta-amyloid-targeting therapies.
Future research will need to investigate the long-term safety and efficacy of PHPD5 in human clinical trials. Additionally, understanding how this peptide interacts with other cellular mechanisms in the brain could pave the way for even more refined treatments. If successful, PHPD5 and similar therapies could not only halt the progression of Alzheimer’s but also restore lost neuronal functions, offering hope for reversing some of the devastating effects of this disease.
Dr. Emer MacSweeney, CEO and Consultant Neuroradiologist at Re:Cognition Health, who was not involved in the study, told Medical News Today: “This research appears to be pioneering in specifically targeting the dynamin-microtubule interaction with the synthetic peptide PHDP5. While other treatments for Alzheimer’s focus on different mechanisms, such as amyloid-beta plaques and tau tangles, targeting the dynamin-microtubule pathway is relatively novel. The researchers have shown in vitro and in vivo evidence of the positive effects of inhibiting this interaction, suggesting this could be the first time it has been targeted in this manner.”
Peptide treatment reaches the brain
Building on earlier findings that demonstrated the efficacy of the synthetic peptide PHPD5 in in vitro studies, researchers advanced their investigation by testing the peptide in a living model. They used transgenic Tau609 mice, a widely recognized model for Alzheimer’s disease research. These mice develop hallmark features of the disease, including tau tangles, neuronal loss, and memory deficits, making them an ideal subject for evaluating the therapeutic potential of PHPD5.
Study Design and Administration
The researchers administered 2 mg of PHPD5 in saline solution intranasally once daily for four weeks. This method was chosen for its ability to deliver therapeutics directly to the brain, bypassing systemic metabolism and efficiently crossing the blood-brain barrier. As a control, another group of Tau609 mice received only the saline solution via the same delivery method.
Cognitive Testing Using the Morris Water Maze (MWM)
To assess the impact of the treatment on memory and learning, the researchers employed the Morris Water Maze (MWM) test, a gold standard for evaluating spatial memory in animal studies. After three weeks of treatment, the mice underwent four training sessions in a circular water trough, 100 cm in diameter and 30 cm deep. The objective was for the mice to locate an escape platform submerged in one quadrant of the trough. This training phase was designed to test their ability to learn and remember the platform’s location.
Following the training sessions, the researchers conducted a memory test in the same water trough, but this time without the escape platform. They measured how much time the mice spent in the quadrant where the platform had been previously located. This provided a clear indication of the mice’s ability to recall the platform’s location, reflecting their spatial memory.
Results and Brain Analysis
After the behavioral testing, the mice were euthanized, and their brains were examined to evaluate the physiological effects of PHPD5. Notably, the researchers found that the peptide successfully crossed the blood-brain barrier and accumulated in the hippocampus, the brain region critical for learning and memory. This finding is significant, as the hippocampus is particularly vulnerable to damage in Alzheimer’s disease, and effective therapeutic delivery to this region is a critical hurdle in neurodegenerative research.
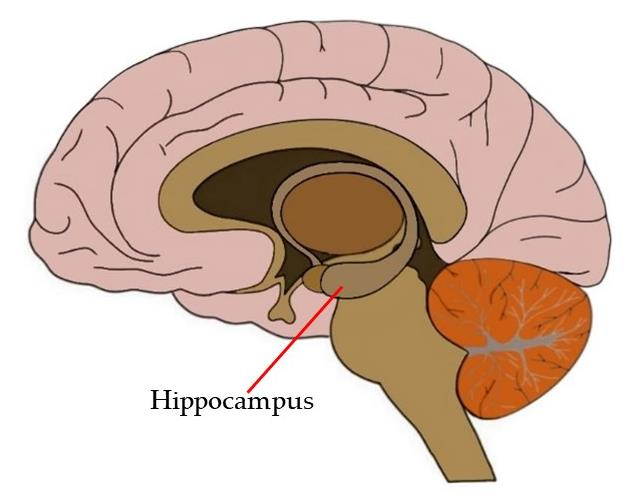
Implications and Future Directions
The results of this study are highly encouraging. The successful delivery of PHPD5 to the hippocampus and its potential to restore learning and memory in Tau609 mice suggest that this peptide could offer a novel therapeutic avenue for treating Alzheimer’s disease and other tau-related neurodegenerative conditions. The intranasal administration route is particularly noteworthy, as it provides a non-invasive and efficient method of drug delivery to the brain. Future research will need to address several key questions. While the results in mice are promising, human trials are necessary to confirm the peptide’s safety, efficacy, and long-term effects. Additionally, researchers must explore the mechanisms by which PHPD5 interacts with tau pathology, as well as its potential impacts on other brain regions affected by Alzheimer’s disease.
If these findings translate to human patients, PHPD5 could represent a groundbreaking advancement in Alzheimer’s treatment—offering hope for not only slowing disease progression but also reversing some of its cognitive and neurological damage. This study exemplifies the importance of innovative research in tackling the complexities of neurodegenerative diseases.
Improvements in learning and memory
The researchers evaluated the effects of PHPD5 treatment on learning and memory by comparing the performance of three groups of mice in the Morris Water Maze (MWM): wild-type (WT) mice, untreated control Tau609 mice, and PHPD5-treated Tau609 mice. This comparison provided a comprehensive assessment of the peptide’s ability to restore cognitive functions impaired by tau pathology.
During the four-day training phase, the WT mice performed the best, demonstrating a 60% reduction in the time required to locate the hidden platform by the fourth session, reflecting their intact learning and memory capabilities. In contrast, the control Tau609 mice showed impaired learning, achieving only a 33% reduction in finding time over the same period, highlighting the cognitive deficits associated with tau accumulation. Remarkably, the PHPD5-treated Tau609 mice displayed significant improvements, with their performance closely mirroring that of the WT mice. By the fourth training session, the treated mice achieved a 55% reduction in the time needed to locate the platform, indicating substantial recovery of learning abilities.
On the fifth day, the researchers assessed memory retention by removing the platform and measuring how much time each group spent in the quadrant where the platform had been located. WT mice spent 36% of their time in the platform’s previous location, demonstrating strong spatial memory retention. PHPD5-treated Tau609 mice spent 33% of their time in the same quadrant, showing near-normal memory recall comparable to the WT group. In contrast, control Tau609 mice spent only 25% of their time in the platform quadrant, underscoring their impaired memory retention. These findings suggest that PHPD5 treatment effectively restored the learning and memory abilities of Tau609 mice to levels like those of healthy WT mice. The treated mice’s ability to improve their performance during training and retain the memory of the platform’s location highlights the peptide’s potential in addressing cognitive deficits caused by tau pathology.
These results align with previous observations that PHPD5 stabilizes tau and facilitates dynamin availability for vesicle recycling, thereby restoring synaptic communication. By mitigating the effects of tau tangles and neuronal dysfunction, the peptide appears to directly address the mechanisms underlying learning and memory impairments. The success of PHPD5 in this experimental setup has far-reaching implications for Alzheimer’s disease research and treatment development.
Future studies should focus on investigating the long-term effects of sustained treatment with PHPD5, conducting human trials to evaluate its safety and efficacy, and exploring its potential as part of combination therapies targeting both tau and beta-amyloid. Additionally, researchers should assess whether PHPD5 could benefit other tau-related neurodegenerative diseases, such as frontotemporal dementia or progressive supranuclear palsy. If the success seen in animal models translates to humans, PHPD5 could represent a breakthrough in Alzheimer’s treatment, offering hope for millions affected by the disease.
A new target for Alzheimer’s treatment?
These are early findings in a new target area for Alzheimer’s treatment, but the study has shown that, in mice, drugs administered intranasally can cross the blood-brain barrier to reach the part of the brain most affected by Alzheimer’s. It has also shown that the synthetic peptide can reverse some of the damage caused by tau in mice genetically engineered to develop Alzheimer’s-like pathophysiology. The research on PHDP5 and its potential to reverse cognitive decline in Alzheimer’s is very encouraging and represents a significant advancement in the search for effective treatments. This could pave the way for new treatments that might prevent or significantly delay the progression of cognitive symptoms in Alzheimer’s.
References:
- Breijyeh Z, Karaman R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules. 2020 Dec 8;25(24):5789. doi: 10.3390/molecules25245789. PMID: 33302541; PMCID: PMC7764106.
- Bondi MW, Edmonds EC, Salmon DP. Alzheimer’s Disease: Past, Present, and Future. J Int Neuropsychol Soc. 2017 Oct;23(9-10):818-831. doi: 10.1017/S135561771700100X. PMID: 29198280; PMCID: PMC5830188.
- Ana R. Monteiro, Daniel J. Barbosa, Fernando Remião, Renata Silva, Alzheimer’s disease: Insights and new prospects in disease pathophysiology, biomarkers and disease-modifying drugs, Biochemical Pharmacology, Volume 211, 2023, 115522, ISSN 0006-2952, https://doi.org/10.1016/j.bcp.2023.115522.
- Chia-Jung Chang, Zacharie Taoufiq, Hiroshi Yamada, Kohji Takei, Takami Tomiyama, Tomohiro Umeda, Tetsuya Hori, Tomoyuki Takahashi, The microtubule-dynamin binding inhibitor peptide PHDP5 rescues spatial learning and memory deficits in Alzheimer’s disease model mice, Brain Research, Volume 1838, 2024, 148987, ISSN 0006-8993, https://doi.org/10.1016/j.brainres.2024.148987.
Product available for research use only.

Leave a comment
Your email address will not be published. Required fields are marked *